
Currently Genomtec is developing its technology in two areas:
1. Mobile platform for POCT genetic (Point-of-Care testing) Identification of Infectious Diseases:
1.1. Genomtec® ID Analyzer.
1.2. Genomtec® ID Respiratory Panel 5-Plex.
2. Technology for Point of Care Companion Diagnostics.
2.1. SNAAT(R) chemistry for mutation detection in Oncology – insertions, deletions and variant analysis (e.g. SNPs).


Genomtec ID is a flagship technological solution currently under development, which will offer patients ultrafast mobile genetic diagnosis utilizing SNAAT®. The Genomtec ID platform makes it possible to run a diagnostic process at point of care, i.e., public clinics, consulting rooms, hospital, pharmacies and emergency wards, without the need of complicated and time-consuming handling by skilled personnel in the laboratory.
The platform includes an analyzer and reaction card with integrated genetic tests and provides multiplexing capability of up to five genetic targets at the same time.
Nowadays, molecular diagnostics requires the user to know how to handle diagnostic equipment and how to correctly prepare the sample. SNAAT® combines mobility with incredible ease of use. The intuitive and simple diagnostic platform can be handled by health care professionals with no laboratory training, i.e., nurse, doctor, paramedic.
Up to 100%
Up to 100%
You only need to apply a single drop of biological material, the test does not require prior sample preparation
The analyzer may be used by health care professionals, including GPs, pediatricians, gynecologists, nurses and paramedics
Cost-effectiveness per test is comparable to other available technologies
Up to 5 targets during 1 test
In the future, the analyzers shall provide anonymous information on the global outbreaks of individual diseases.
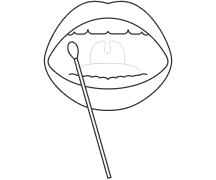

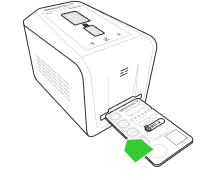


Genomtec ID – Mobile platform for genetic diagnostics
