
Currently, laboratory genetic diagnostics of SARS-CoV-2 utilizes the RT-PCR method, namely its stype Real-Time Reverse Transcription PCR. In this method, virus genetic material (RNA) is transcribed to complementary DNA (so-called cDNA) by an enzyme, reverse transcriptase combined with a cDNA multiplication stage using another enzyme – polymerase. The detection mechanism is usually based on molecular probes or fluorescent dyes. We can assume that the average lab time required for Real-Time RT-PCR reaction for detecting such pathogen as SARS-CoV-2 is 90 minutes, and often can be longer, depending on the applied enzymes and the performance of Real-Time PCR device.
The Real-Time RT-LAMP makes it possible to analyze the same sample in around 30 minutes using the same laboratory equipment.
The Genomtec products listed below have been market authorised (European Union – CE-IVD). For each product there is a downloadable document list (if it is authorized for sale).
The Genetic tests Genomtec SARS-CoV-2 Duo Kit (cat. no. GA00B) and Genomtec SARS-CoV-2 Direct-RT-LAMP Kit (cat. no. GA00C) detect multiple coronavirus variants (B.1.1.7, Breton [hCoV-19/France/BRE-IPP04233/2021], P1, B. 1.351, B.1.525, P.3, B.1.427, B.1.429 and B.1.617).
Genomtec also launched the Genomtec® SARS-CoV-2 EvaGreen® Direct product line. These products do not use standard lab purification of genetic material. This is replaced by a lysis combined with purification due to the application of a special buffer and an increased temperature. Consequently, the nucleic acid isolation stage is replaced by a fast and simple procedure, which saves time and reduces the total diagnostic cost.
Addtionally to Genomtec® RT-LAMP-Direct we offer Saliva Sample Collector Device.

Instruction how to use the world’s first genetic diagnostic test for the detection of SARS-CoV-2 directly from a saliva sample: Genomtec® SARS-Cov-2 EvaGreen® Direct-RT-LAMP CE-IVD Kit.

Saliva sample, or throat or nasopharyngeal dry swab is obtained
Collected specimen (saliva or dry swab) is transferred to the diagnostic laboratory within 24 hs

Add LysBuffer to saliva or dry swab and heat for 5 min. at 95oC to prepare RNA-enriched supernatant. Saliva / dry swab sample preparation stage with LysBuffer takes 5 minutes onto the heat block (pipetting excluded)
Purified RNA is simultaneously reverse transcribed to cDNA and amplified in LAMP technology
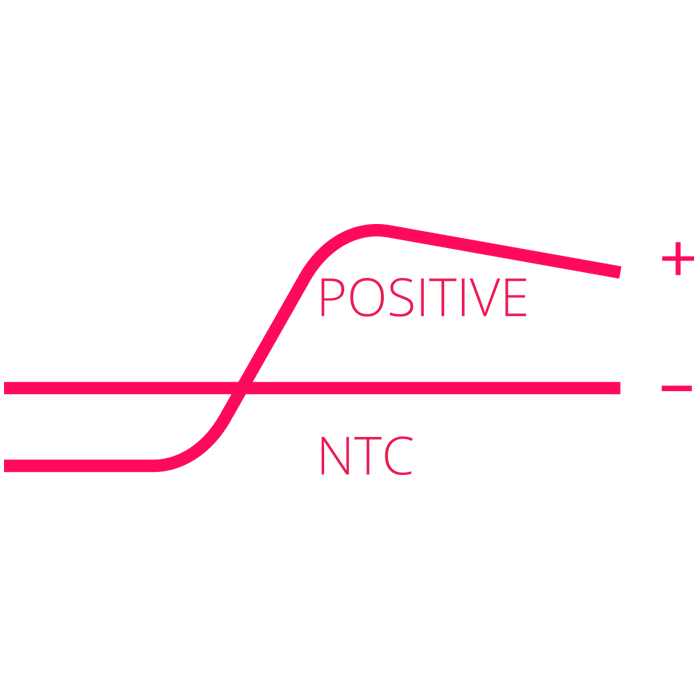
Positive clinical samples can be detected in as little as 20 minutes – when fluorescence exceeds threshold
The Agency for Health Technology Assessment and Tariff System has issued a 2.0 update of the recommendations “Laboratory diagnostics for SARS-CoV-2”.
The recommendations were prepared by the Scientific Council and the Expert Panel based on the expert consensus, resulting from the current epidemiological situation and reviews of scientific articles on SARS-CoV-2 diagnostic methods.
The document once again indicates that genetic tests offer an advantage when confirming infection at an early stage, before clinical symptoms occur, while antigen tests still show negative results.
The recommendations for molecular diagnostics confirm that “fast RT-PCR genetic tests or isothermal RT-LAMP tests” can be used. The quality of testing is monitored by the laboratory enlisted on the Ministry of Health “COVID laboratories” list.
The entire document can be downloaded here: Download PDF
| Document name | Version | Published | |
|---|---|---|---|
| Analysis certificate | 0000C | 2020-12-16 | Download |
| Analysis certificate | 0000D | 2021-02-05 | Download |
| Analysis certificate | 0000E | 2021-03-04 | Download |
| Analysis certificate | 0000F | 2021-05-17 | Download |
| Analysis certificate | 0000G | 2021-06-09 | Download |
| Analysis certificate | 0000H | 2021-09-02 | Download |
| Analysis certificate | 0000I | 2021-12-03 | Download |
| Analysis certificate | 0000J | 2021-12-21 | Download |
| Analysis certificate | 0000K | 2022-02-02 | Download |
| Analysis certificate | 0000L | 2022-02-15 | Download |
| Document name | Version | Published | |
|---|---|---|---|
| Product card | PI00CUKrE | 2022-01-07 | Download |
| User manual | IFU00CUKrE | 2022-01-07 | Download |
| Information brochure | MM00CUKrC | 2022-01-14 | Download |
| Fast portable lab | 2021-08-02 | Download |
|
| Variants recognition report | 2021-11-30 | Download |
| Document name | Version | Published | |
|---|---|---|---|
| General Terms and Conditions of Sale and Services | 01.2020 | 2020-11-23 | Download |
