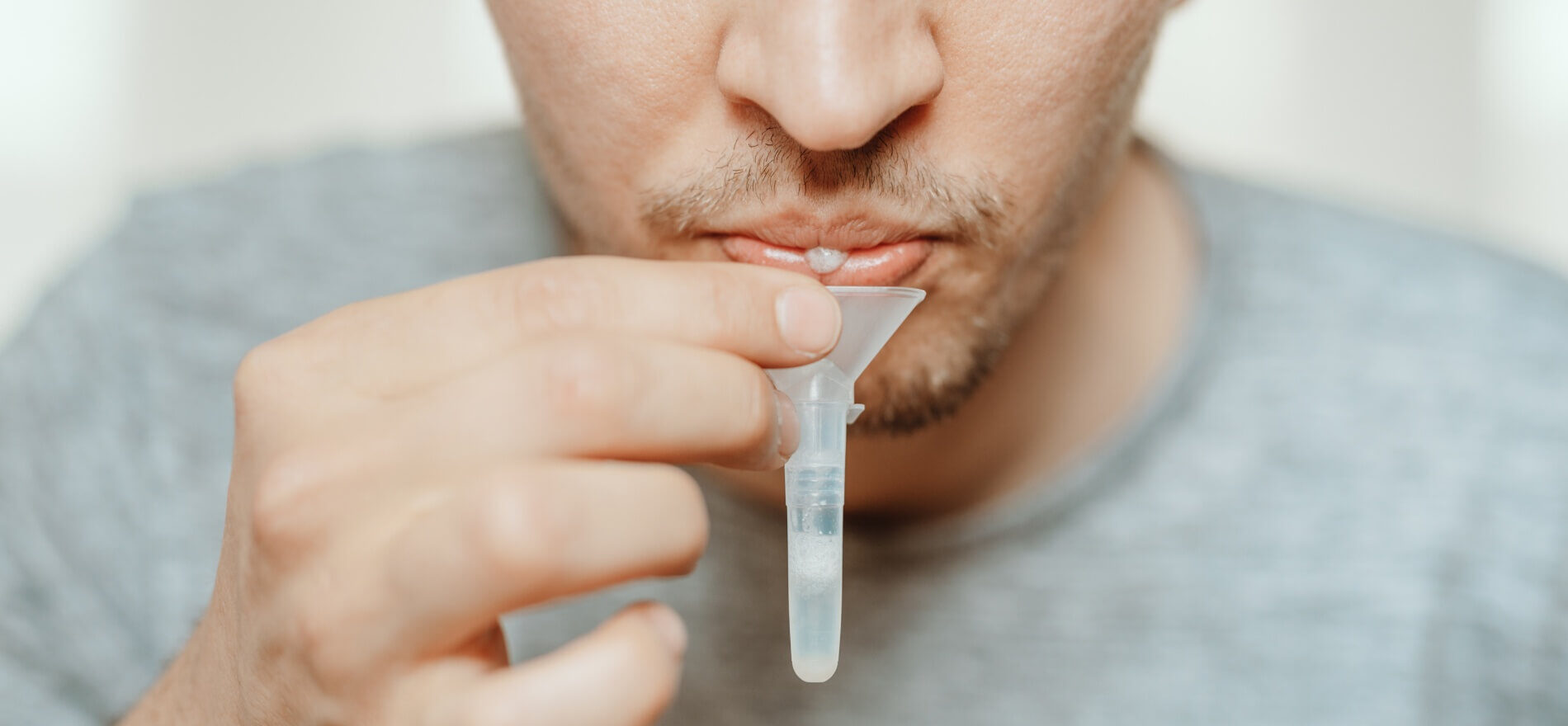
Genomtec S.A., listed on the NewConnect market at Warsaw Stock Exchange, a company developing medical technology applicable in segment of clinical diagnostics at the point of care testing – POCT, has registered and launched in the European Union market the Genomtec® SARS-Cov-2 EvaGreen® Direct-RT-LAMP CE-IVD Kit (RT-LAMP Direct Kit), which enables identification of SARS-CoV-2 infection from the patient’s saliva. The innovative test will provide patients with greater comfort due to lack of swabbing, e.g. from the nasopharynx. The possibility of testing using saliva will also reduce the risk of virus’ contraction by the personnel collecting the sample, additionally sampling does not require specialized skills. Utilization of the Direct-RT-LAMP Kit makes it possible to receive test’s results in just several minutes from the time the sample was received by the laboratory. The ultrafast test for COVID-19, which detects all currently identified SARS-CoV-2 variants, will be performed at the Dolmed laboratory in Wroclaw already in May.
The diagnostic Genomtec® SARS-Cov-2 EvaGreen® Direct-RT-LAMP CE-IVD Kit employs direct RT-LAMP technology, while maintaining a high degree of diagnostic parameters, offers patients and medical staff a comfort resulting from the biological material collection in the form of saliva. Importantly for laboratories, due to the use of a special lysis and sample purification technology, the time needed to perform the full diagnostic procedure (from sample to result) has been reduced by almost 60% compared to the currently used RT-PCR tests requiring standard purification of genetic material. Consequently, the cost of the entire diagnostic protocol has been significantly reduced, as it is unnecessary to use nucleic acid isolation kit for the purification of SARS-CoV-2 virus genetic material.
Registration of the new Genomtec product was enabled by results of a comparative study carried out at the Central Clinical Hospital of the Ministry of Interior and Administration in Warsaw (MSWiA Hospital). Genomtec’s internal study results conducted on the kit’s pilot version utilizing saliva samples indicated that it has one of the lowest detection limits in the world, being equal to only two virus copies in the reaction mixture.
– By elimination of the laboratory nucleic acid purification stage from biological material, we have shortened the processing time and simplified the entire diagnostic process, which also reduces its cost of labour. Our new genetic tests from saliva will be able to increase the laboratories’ diagnostic throughput by several times, while providing comfort to the patient and medical staff. Our innovative Direct tests will become an effective tool in the fight against pandemic and controlling its population transmission. The development of new Genomtec’s products in the field of laboratory medicine is the key element of our strategy. It also confirms our competency and high level of know-how at Genomtec’s team. – said Miron Tokarski, Co-founder and President of Genomtec.
Patients will be able to perform a diagnostic test with the Genomtec Direct-RT-LAMP Kit already in May in the laboratory of the Dolnośląskie Centrum Medyczne DOLMED in Wroclaw, located at Legnicka 40 street. The laboratory is the reference facility for products offered by Genomtec.
– Introduction of genetic tests in the RT-LAMP technology, which constitute undoubted market innovation, and the start of cooperation with DOLMED, which will use our diagnostic solutions, may be of significant importance for Genomtec economic activities and revenues generated – Michał Wachowski, member of the Board and CFO Genomtec.
Genomtec tests are compatible with the devices already owned by laboratories performing tests in the Real-Time PCR technique, which may aid in releasing the diagnostic bottleneck without additional investment in the infrastructure and new personnel hires. Currently offered Genomtec’s tests can increase the throughput of any laboratory by almost 50%, compared to the fastest RT-PCR tests, or save 33% diagnostic time needed to perform the same number of genetic analyses, a feature so important during the pandemic waves.
The product will be manufactured in Poland at Genomtec S.A. facility in Wrocław. Genomtec’s clients will have the option to purchase additionally to the diagnostic kit a disposable saliva collector / collection device.
Genomtec’s flagship solution – the Genomtec ID is a mobile diagnostic system employing microfluidic system and the proprietary, patented SNAAT® technology. This innovative diagnostic system will be able to carry out several tests from one sample simultaneously, including causal determination of respiratory tract infections or sexually transmitted diseases, even in 15 minutes. Genomtec ID will be used for point of care diagnostics, i.e., in doctor’s offices, emergency department, and even a pharmacy.
Additional information available at:
|
Genomtec S.A. Magdalena Kicińska +48 604 201 230 |
Genomtec SA Charles Winiarski +44 7972 406 384 |
About Genomtec S.A.
Genomtec is an innovative medical technology company dedicated to the development and commercialization of a mobile molecular diagnostics platform for detection of various infectious diseases, and also rapid laboratory diagnostic tests used, among others, in the detection of COVID-19 disease caused by the SARS-CoV-2 virus.
The company’s flagship project is the Genomtec ID mobile IVD platform. The analyser is uniquely placed among Point-Of-Care (POC) products worldwide. It will allow for a quick and precise clinical molecular analysis outside the standard laboratory setting, without the need to involve qualified laboratory personnel. The system uses microfluidic technology and the proprietary, patent-protected SNAAT® isothermal technology. Appropriate design of the system enables the process to be carried out in record time, i.e. even in 15 minutes, with the diagnostic parameters equal to, and in some cases exceeding, the quality of PCR laboratory tests.
The development and manufacturing process is executed in close cooperation with international CMO (Contract Manufacturing Organization) companies. According to the assumptions of the Genomtec Management Board, the commercialization of the flagship solution will take place in the first half of 2022.
Manufacturing of Genomtec® SARS-CoV-2 EvaGreen® laboratory tests in RT-LAMP technology is based in Poland, with current EU market authorisation (CE-IVD), and product registration started in other regulatory jurisdictions outside Europe.
Genomtec was founded in 2016. The seat of Genomtec SA is located in Wroclaw.